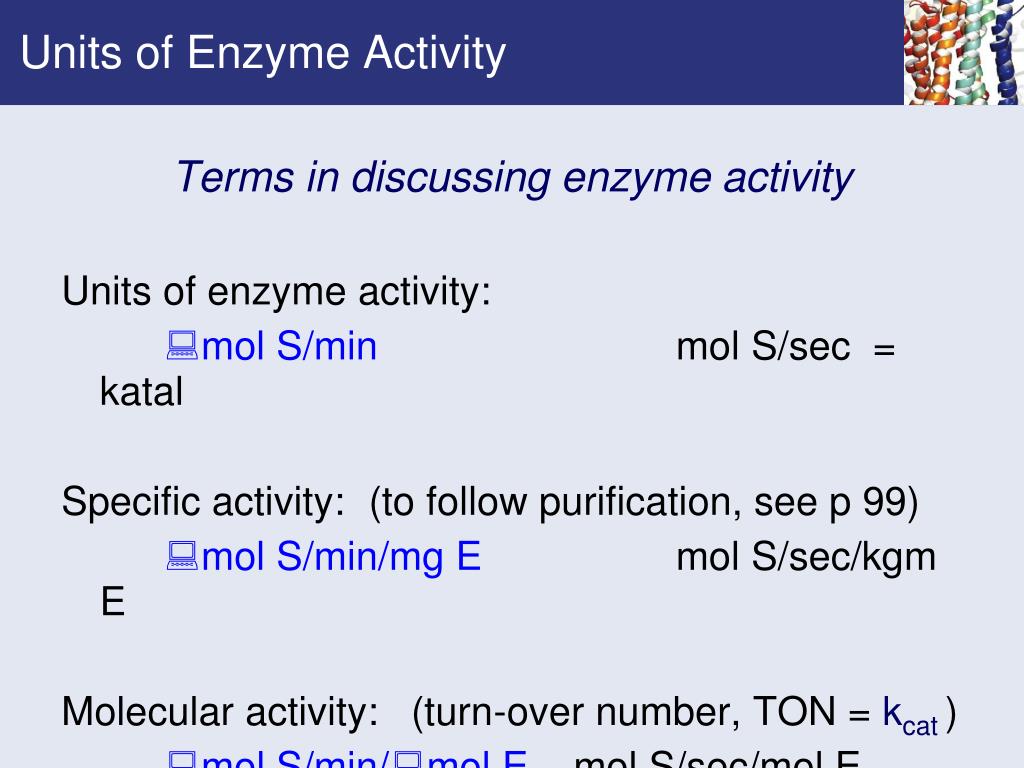
Hill Enzyme Kinetics . The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. The hill equation is an equation used in biochemical characterization. The hill equation is derived from the analysis of the following binding equilibrium: Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. In biochemistry, the binding of a ligand to a macromolecule is. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry.
from www.slideserve.com
Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. The hill equation is an equation used in biochemical characterization. The hill equation is derived from the analysis of the following binding equilibrium: Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. In biochemistry, the binding of a ligand to a macromolecule is.
PPT Chapter 13 Enzyme PowerPoint Presentation, free download
Hill Enzyme Kinetics The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. In biochemistry, the binding of a ligand to a macromolecule is. E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. The hill equation is an equation used in biochemical characterization. The hill equation is derived from the analysis of the following binding equilibrium: Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose.
From www.worthington-biochem.com
Enzyme Energy Levels Worthington Biochemical Hill Enzyme Kinetics The hill equation is derived from the analysis of the following binding equilibrium: In biochemistry, the binding of a ligand to a macromolecule is. The hill equation is an equation used in biochemical characterization. Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. The hill equation (see. Hill Enzyme Kinetics.
From www.researchgate.net
Sigmoidal of PpOTC. The graph shows the saturation curve of Hill Enzyme Kinetics Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. In biochemistry, the binding of a ligand to a macromolecule is. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. The hill equation is an equation used in biochemical characterization. Kd is the equilibrium dissociation. Hill Enzyme Kinetics.
From www.slideserve.com
PPT Allosteric regulation of enzyme activity PowerPoint Presentation Hill Enzyme Kinetics The hill equation is an equation used in biochemical characterization. In biochemistry, the binding of a ligand to a macromolecule is. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. Kd is the equilibrium dissociation constant, which. Hill Enzyme Kinetics.
From www.slideserve.com
PPT Chapter 6.3 Enzyme PowerPoint Presentation, free Hill Enzyme Kinetics The hill equation is an equation used in biochemical characterization. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the. Hill Enzyme Kinetics.
From www.slideserve.com
PPT Chapter 13 Enzyme PowerPoint Presentation, free download Hill Enzyme Kinetics Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. In biochemistry, the binding of a ligand to a macromolecule is. E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. The hill equation is derived from the analysis of the following binding equilibrium: The hill equation (see below) is commonly used. Hill Enzyme Kinetics.
From www.slideserve.com
PPT Globins PowerPoint Presentation ID1348492 Hill Enzyme Kinetics Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. In biochemistry, the binding of a ligand to a macromolecule is. The hill equation is an equation used in biochemical characterization. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a. Hill Enzyme Kinetics.
From www.researchgate.net
Enzyme of the AcPDO. (A) activity vs. substrate Hill Enzyme Kinetics E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. In biochemistry, the binding of a ligand to a macromolecule is. Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in. Hill Enzyme Kinetics.
From www.slideserve.com
PPT Allosteric regulation of enzyme activity PowerPoint Presentation Hill Enzyme Kinetics The hill equation is derived from the analysis of the following binding equilibrium: Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The hill equation (see below) is commonly used to study. Hill Enzyme Kinetics.
From www.slideserve.com
PPT Allosteric regulation of enzyme activity PowerPoint Presentation Hill Enzyme Kinetics E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. The hill equation is an equation. Hill Enzyme Kinetics.
From enzymkinetics.com
Enzyme BestCurvFit Software (EZFit, Perrella), Hill Enzyme Kinetics The hill equation is derived from the analysis of the following binding equilibrium: Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. In biochemistry, the binding of a ligand to a macromolecule is. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in. Hill Enzyme Kinetics.
From www.youtube.com
MichaelisMenten YouTube Hill Enzyme Kinetics The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. The hill equation is derived from the analysis of the following binding equilibrium: The hill equation is an equation used in biochemical characterization. In biochemistry, the binding of a ligand to a macromolecule is. Kd is the equilibrium dissociation constant, which. Hill Enzyme Kinetics.
From fity.club
Enzyme Hill Enzyme Kinetics Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. Kd is the equilibrium dissociation constant, which would be named ec 50 (dose. Hill Enzyme Kinetics.
From www.researchgate.net
DPP8 and DPP9 enzyme with SLRFLYEG and 1G244 reveal allosteric Hill Enzyme Kinetics The hill equation is an equation used in biochemical characterization. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. The hill equation is derived from the analysis of the following binding equilibrium: Kd is the. Hill Enzyme Kinetics.
From jackwestin.com
1a Enzymes MCAT Content Hill Enzyme Kinetics In biochemistry, the binding of a ligand to a macromolecule is. Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. The hill equation is an equation used in biochemical characterization. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a. Hill Enzyme Kinetics.
From www.chegg.com
Solved 3. Hill Number Enzyme Consider an enzyme Hill Enzyme Kinetics Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. In biochemistry, the binding. Hill Enzyme Kinetics.
From guruguruips.blogspot.com
Ki Kd Ec50 Guru IPS Hill Enzyme Kinetics The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. E + nl ←→kd eln kd = [e] [l]n [eln], (1) where e. In biochemistry, the binding of a ligand to a macromolecule is. Examining enzyme kinetics is critical for understanding cellular systems and for using enzymes in industry. The hill. Hill Enzyme Kinetics.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Hill Enzyme Kinetics Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. The hill equation is derived from the analysis of the following binding equilibrium: The hill equation is an equation used in biochemical characterization. The hill equation (see below) is commonly used to study the kinetics of reactions that. Hill Enzyme Kinetics.
From www.lecturio.com
Enzyme Inhibition Concise Medical Knowledge Hill Enzyme Kinetics In biochemistry, the binding of a ligand to a macromolecule is. Kd is the equilibrium dissociation constant, which would be named ec 50 (dose for 50% effect) in the case of classical dose. The hill equation (see below) is commonly used to study the kinetics of reactions that exhibit a sigmoidal behavior. The hill equation is an equation used in. Hill Enzyme Kinetics.